The treatment of anatomical waste is a major concern for healthcare facilities and laboratories. Proper management is essential to protect staff health, comply with current regulations, and minimize environmental impact. It is therefore crucial to clearly distinguish between anatomical specimens and anatomical waste, as their methods of treatment, storage, and transport differ depending on their nature and risk level.
But what actually happens to human anatomical specimens, and how is their disposal regulated in healthcare facilities?
Human anatomical specimens
Human anatomical specimens, as defined by Article R.1335-9 of the French Public Health Code, refer to organs or limbs that are easily identifiable by a non-specialist. This includes, for example, an arm, a leg, a heart, or any other whole organ.

These specimens require strict disposal to prevent any health risks and to comply with legislation. According to Article R.1335-11 of the French Public Health Code, they must be incinerated in an authorized crematorium, outside of public visiting hours, to ensure safety and confidentiality. Adhering to these regulations also helps protect the environment, as authorized crematoriums are equipped to minimize pollutant emissions and process waste in accordance with environmental standards.
In practice, anatomical specimens must be stored at a controlled temperature (0–5°C) or frozen if they cannot be disposed of immediately. They must be packaged in rigid, leak-proof containers approved for transport, in order to prevent any risk of leakage or contamination.
Human anatomical specimAnatomical wasteens

Anatomical waste includes fragments of tissues, organs, or limbs that are not easily identifiable by a non-specialist. They can be solid, liquid, or soft, and may include samples obtained from biopsies, punctures, or surgical procedures.
These wastes pose a significant health risk, as they may be infectious or contaminated. For this reason, they must systematically be directed to the Healthcare Waste stream. This system allows for the safe packaging, storage, transport, and disposal of such waste, in compliance with legal standards and best safety practices for both staff and the environment.
Special cases
Certain situations require special attention. For example, placentas—unless they are used for therapeutic or scientific purposes—must be considered anatomical waste and directed to the Healthcare Waste stream. If the facility performs pretreatment through disinfection, it is essential to ensure that any excess liquid produced can be absorbed by other waste to be treated, in order to minimize health risks and facilitate transport and disposal.
La prise en charge des corps d’enfants décédés, des enfants sans vie et des fœtus est strictement encadrée par la circulaire du 19 juin 2009. Les établissements doivent s’assurer que ces corps soient manipulés avec dignité, stockés dans des conditions appropriées et transportés selon les protocoles autorisés, avec une traçabilité complète.
Main management steps
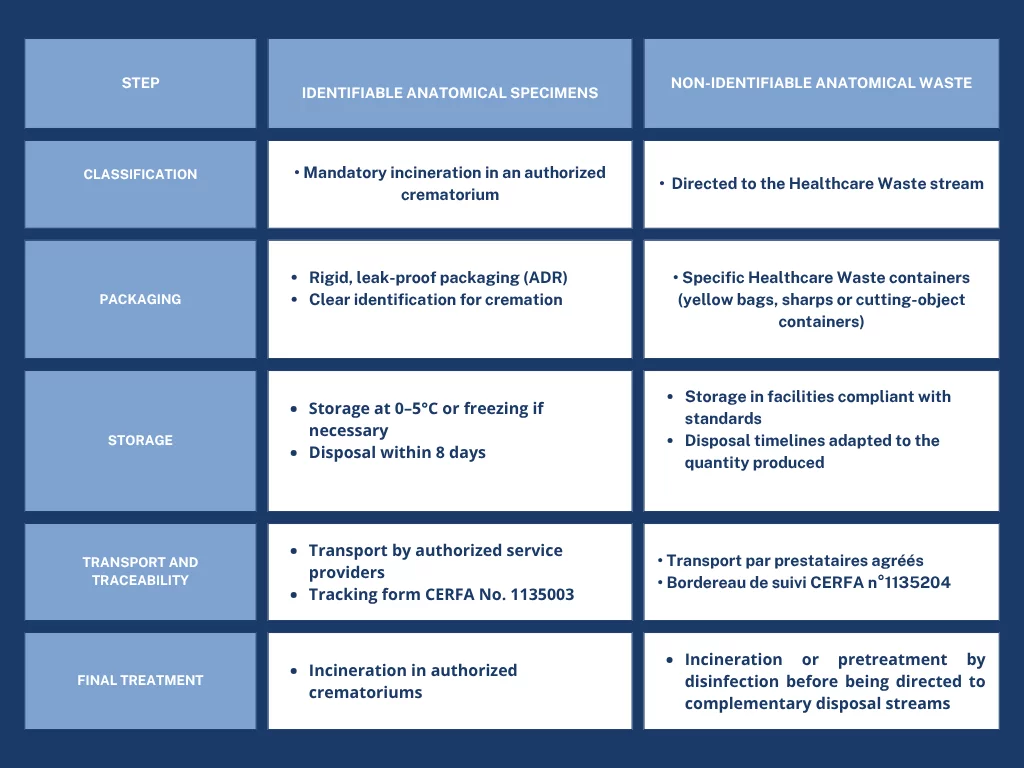
The management of human anatomical specimens and waste follows several key steps, allowing for the safe classification, packaging, storage, transport, and treatment of waste. The table below summarizes these steps, distinguishing between identifiable anatomical specimens and non-identifiable waste:
Technologies and methods to enhance treatment safety

For facilities aiming to optimize the safety and efficiency of anatomical and Healthcare Waste management, certain technologies can complement the best practices described above.
For example, Tesalys’ STERIPLUS™ and STERISHRED® systems allow waste to be shredded and sterilized directly on-site, thereby reducing waste volume, eliminating infectious risks, and facilitating traceability.
These solutions provide a concrete example of implementing best practices, ensuring that hazardous waste is treated safely and in compliance with regulations, while minimizing environmental impact. They enhance the existing system but do not replace the application of the traditional management steps: classification, packaging, storage, and transport remain essential.
Conclusion – Safety and Compliance Above All
The management of anatomical specimens and waste relies on key steps: classification, packaging, storage, and final treatment. Distinguishing identifiable specimens from non-identifiable waste allows for the adoption of the appropriate disposal route: authorized cremation or secure Healthcare Waste treatment.
By applying these best practices and using innovative solutions such as those from Tesalys, facilities ensure safety, regulatory compliance, and a reduction of infectious risks.
Now you know everything for the safe and responsible management of anatomical waste!